BREAKING: DNA contamination in Australian mRNA Covid shots up to 145 times regulatory limit, report shows
The first independent testing of Australian vials confirms findings from the US, Canada and Germany, highlighting that oncogenic and genomic integration risks are a global concern
Synthetic plasmid DNA contamination has been detected in Australian vials of Pfizer and Moderna Covid vaccines at levels of between seven to 145 times the allowable limit, a new report shows.
The independent testing of three modified RNA (mod-RNA) vaccine vials, including lots for children and adults, was commissioned to provide evidence in a Federal Court lawsuit over the validity of the regulatory status of the vaccines.
The case, brought by legal firm PJ O’Brien & Associates, alleges that the vaccines contain unlicenced genetically modified organisms (GMOs) in the form of synthetic DNA contamination and mod-RNA-LNP complexes which could pose an untested safety risk, including the potential for DNA integration into the human genome.
In an affidavit provided to legal firm PJ O’Brien & Associates, molecular virologist Dr David Speicher said that the amount of synthetic DNA he detected in all three Australian vials “far exceeded” the allowable regulatory limit set by the Therapeutic Goods Administration (TGA).
Given scientific evidence suggesting that synthetic DNA can enter the cell nucleus and potentially integrate into the human genome, “it is important to investigate whether integration can take place in primary cells in the vaccinated population,” Dr Speicher said.
The Australian testing confirms independent lab findings of high levels of residual DNA in mod-RNA Covid vaccines from Germany, the US and Canada, highlighting that this is a global concern.
The report
Residual synthetic DNA is a byproduct from the mod-RNA vaccine manufacturing process, and is allowed under TGA regulations in levels of up to 10 nanograms (ng) per vaccine dose, and in fragment sizes of up to 200 base pairs (bp).
The TGA denies that Covid mod-RNA vaccines are contaminated with synthetic DNA above 10ng per dose, but as high levels had been detected in vials from other regions, the legal team behind the GMO case commissioned this study to determine residual synthetic DNA levels in Australian vials.
PJ O’Brien & Associates arranged for shipment of three vials with chain of custody, one Moderna and two Pfizer, to Dr Speicher’s lab at the University of Guelph in Canada. The vials were shipped on dry ice and stored in the lab fridge on arrival. The Pfizer vials had tamper seals intact, while the Moderna vial had been half-used.
Dr Speicher used two methods to test for residual DNA levels - fluorometry and qPCR - each with its own advantages.
qPCR is the method preferred by regulators. It captures lower readings of DNA because it can fail to pick up small fragments of DNA below 200bp, and it measures less than 1% of the residual DNA plasmid, with the other 99% being extrapolated mathematically. This means the reading has greater repeatability, but it gives a less complete picture.
A Moderna patent (2014) related to “removal of DNA fragments in [the] mRNA production process” acknowledges that the qPCR method of quantitating residual DNA only detects some target DNA molecules, but “does not measure all other smaller DNA molecules that are partially digested” by the enzyme used to break them down for the filtration process.1
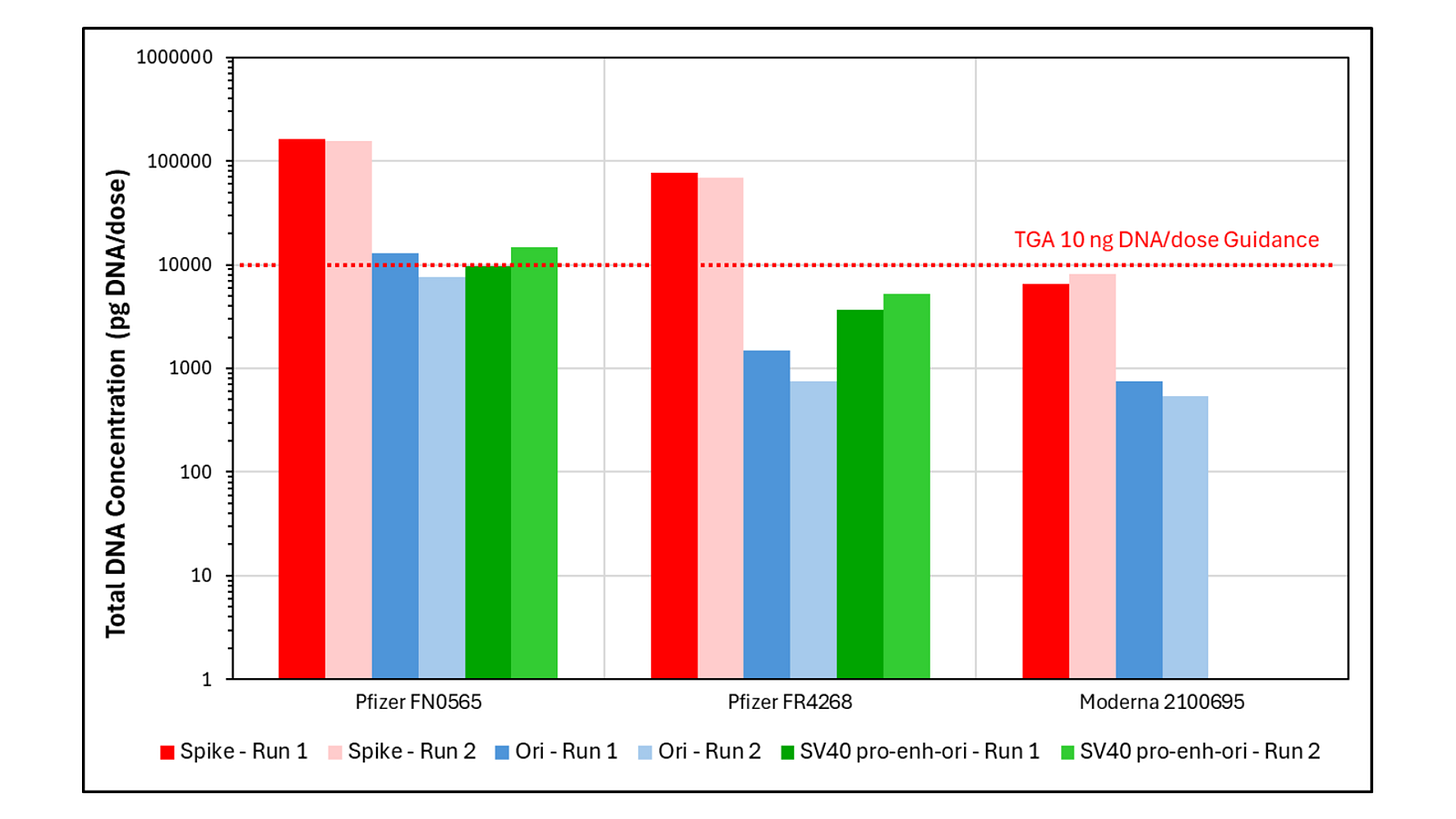
Using qPCR, Dr Speicher detected synthetic DNA up to 15-fold above the TGA’s limit in both Pfizer lots, but the Moderna lot was compliant.
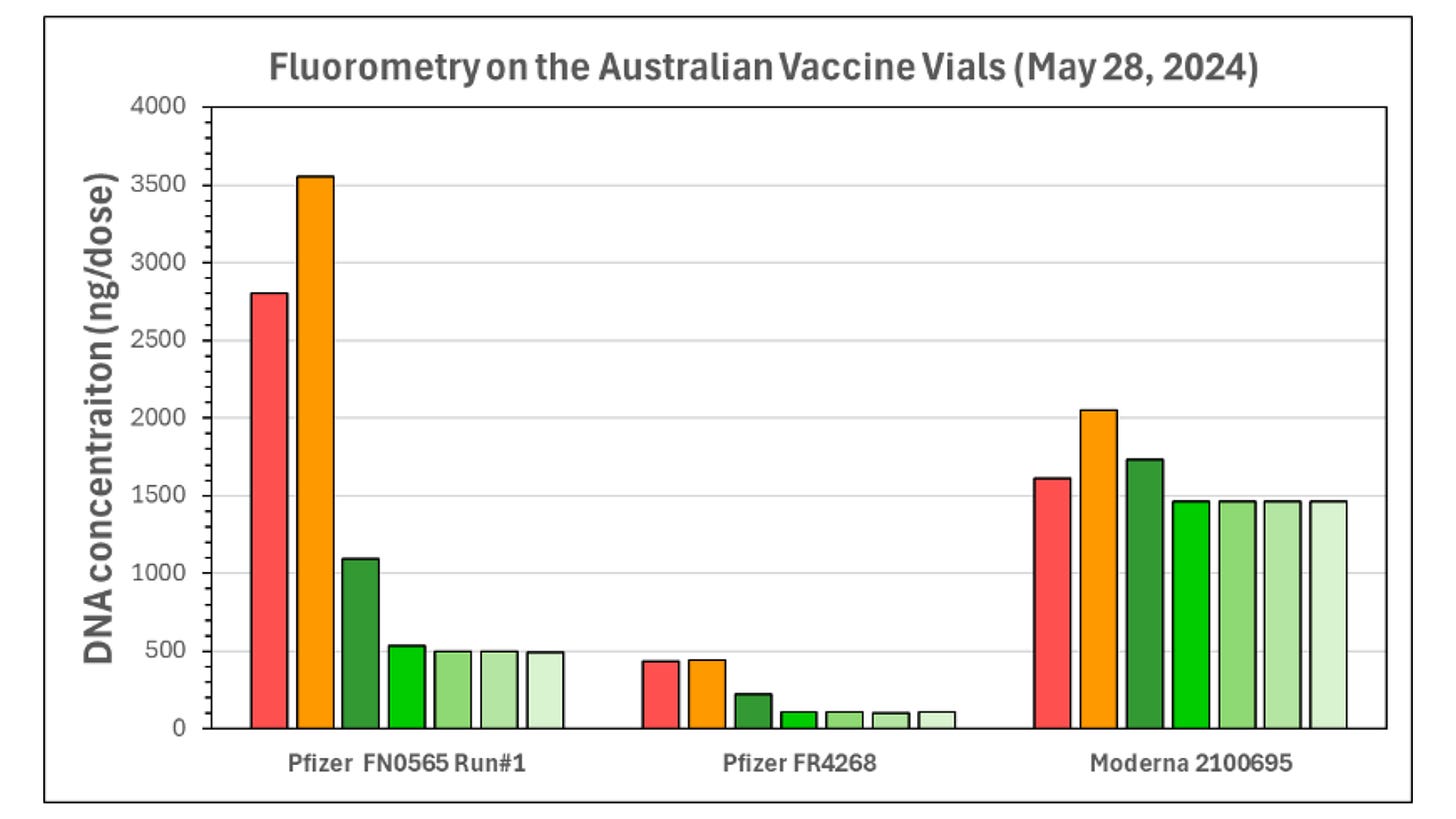
To prepare for fluorometry testing, Dr Speicher boiled the vaccines to dissolve the lipid nanoparticles (LNPs) encapsulating both the mod-RNA and residual synthetic DNA. This allowed for increased DNA yield in the reading.
However, there is the potential for “cross talk,” where mod-RNA can be accidentally included in the reading. To reduce cross talk, Dr Speicher treated the samples with an enzyme called RNase A to degrade the mod-RNA, ensuring that it would not be picked up in the DNA reading.
Using fluorometry, Dr Speicher detected seven to 145-fold more synthetic DNA than the TGA’s 10ng limit. All vials exceeded the limit, with Moderna having the highest DNA load of 1460ng per dose.
Dr Speicher detected three types of residual synthetic DNA in the vaccines: spike protein, ‘ori’ (short for the origin, where the synthetic plasmid begins to be read for copying) and the gene therapy SV40 enhancer/promoter sequence. Pfizer and Moderna contained DNA from the spike and ori, but only Pfizer contained the SV40 enhancer/promoter (not to be confused with the whole Simian Virus 40, which was not present.)
The spike protein DNA detected in the Pfizer vaccines was “the highest concentration levels seen in vials independently tested globally to date,” said Dr Speicher, prompting him to run the tests a second time to make sure it wasn’t an erroneous reading.
Dr Speicher also used vials from a previous study of mod-RNA Covid vaccines as controls to rule out any possibility of contamination or other sources of error. “The results were repeatable suggesting the result is true and valid,” he stated.
SV40 enhancer/promoter
Dr Speicher explained in his affidavit that the SV40 enhancer/promoter is “is known to promote nuclear localization,” meaning that it can drag DNA fragments into the nucleus of the cell after being delivered to the cytoplasm by LNPs.
Once in the nucleus, the probability of genomic integration “greatly increases” as compared to traditional vaccines, where any residual DNA is not packaged in LNPs nor accompanied by a nuclear localising sequence.
Dr Speicher and other scientists have also pointed out that this SV40 sequence could pose an oncogenic risk due to its effect on tumor suppressor gene p53.
Controversially, Pfizer “chose not to mention” the SV40 enhancer/promoter on the residual DNA map submitted to regulators as it should have done, emails obtained under freedom of information show.
Genomics scientist Kevin McKernan was the first to discover the SV40 enhancer/promoter in the Pfizer vaccine, in early 2023. He documented his findings in a preprint and alerted the Food and Drug Administration (FDA).
Since McKernan made the presence of the SV40 enhancer/promoter known, regulators, including the TGA, have issued statements to the effect that the sequence is non-functional and poses no safety risk.
Nevertheless, internal emails show that at least one regulator, Health Canada, is working to get the SV40 enhancer/promoter sequence removed from Pfizer’s mod-RNA vaccine.2
Limitations
Dr Speicher acknowledged several limitations to the report. Though the vials were cool when they arrived to his lab, the dry ice had evaporated and no temperature was recorded at handover.
However, Dr Speicher told me that it is unlikely that this would have any effect on the DNA levels in the vaccines.
“We know that DNA is stable at room temperature for months. The shipping time would have very little negative effect on the DNA levels,” he said.
While breaking the cold chain “could make the LNPs less stable and begin to degrade the modRNA” and would therefore invalidate the vaccines for use in humans, it “would not significantly degrade or change the DNA loads,” said Dr Speicher.
The Moderna vial was not tamper-sealed, leaving open the possibility of external contamination. This is unlikely though, said Dr Speicher, as a person tampering with the vial would have to contaminate the vaccine with the exact same series of DNA sequences detected in the other independent studies around the world, an unlikely theory that McKernan refers to as the ‘elf on the shelf’.
Once the vials arrived in Canada, “the shipping container was opened by me, documented with time and date, and placed in a secure fridge that can only be accessed by myself,” said Dr Speicher.
Dr Speicher also identified some variability between the results from the two flourometry runs, which he attributed to the “difficulty in pipetting LNPs due to aggregations and settling of LNPs.”
Regardless, Dr Speicher emphasised that these results “clearly show that the vials from Australia have more than 10ng per dose.”
“It’s not a matter of whether or not the vaccines have more DNA than 10ng per dose but a question of how much more do they contain,“ he said.
Regulator dismisses findings
In response to independent studies of residual DNA contamination date finding residual synthetic DNA at levels over the allowable limit, the TGA says that the mod-RNA Covid vaccines are “not contaminated,” and denies that the studies are valid. The TGA says that the presence of residual DNA, including the SV40 enhancer/promoter in the mod-RNA Covid vaccines poses no safety risk, and it says that the delivery of synthetic DNA to cells all around the body in LNPs is immaterial.
See the TGA’s full arguments below.
Australian drug regulator goes on record: Pfizer mRNA shots 'not contaminated'
The Therapeutic Goods Administration (TGA) denies that the Pfizer mRNA Covid vaccine is contaminated, as at least four independent labs around the world claim to have detected plasmid DNA contamination in vials of the mRNA Pfizer and Moderna shots, most well over regulatory limits.
In response to Dr Speicher’s affidavit detailing his testing of three Australian mod-RNA vaccine vials, the TGA determined the findings are “not reliable.”
A TGA spokesperson referred me to the international guideline adopted by regulators to determine whether testing methods are reliable and accurate, stating,
“The TGA cannot tell from Dr Speicher’s affidavit whether he has validated his method according to this guideline, nor whether he has used a suitably characterised reference standard. There is no information in the affidavit that Dr Speicher has validated the method using RNAse according to the validation guidelines.
“Regulatory testing is conducted within tightly controlled frameworks that ensure traceability and certainty about the integrity and provenance of test samples.
“It appears that Dr Speicher only used three vials of unknown provenance, one of which he notes had been opened by the time it reached him. All three vials had expired when the testing was conducted. The “chain of evidence” provided in the affidavit only covered 4 hours of time and there is no temperature log with the samples.
“Given the lack of controls that could ensure the accuracy of the test methods used and given considerable uncertainty around sample integrity and provenance, the results presented in Dr Speicher’s affidavit are not reliable.”
Several of the concerns raised by TGA are limitations already addressed above, namely the use of alternative methods for measuring DNA loads, lack of temperature record on arrival of the vials, and the open Moderna vial.
The TGA is correct that Dr Speicher’s affidavit does not contain the chain of custody, however PJ O’Brien & Associates advised that chain of custody is documented and included in the prosecution brief.
Dr Speicher acknowledges the TGA’s adopted guideline but said that “there is no compendial standard for testing DNA inside LNPs.” This is because the approved method of testing measures DNA levels in the ‘mixture’ before packaging it in LNPs, but not in the final drug product as administered, once the DNA has been enveloped in the LNPs.
“The work was done in a research laboratory following good laboratory practice and show important preliminary findings on the vials that need to be confirmed by an independent lab under forensic conditions,” Dr Speicher said.
Oncogenic and genomic integration risks
While the TGA assures that the mod-RNA Covid vaccines are compliant with with regulatory guidelines on DNA limits, Dr Speicher’s affidavit highlights that the guidelines “do not account for multiple dosing of the same vaccine or platform, the risk of regulatory sequences [such as the SV40 enhancer/promoter], integration of small DNA fragments (7bp to 200 bp), or nuclear entry/integration.”
“While the number of these fragments entering a cell is unknown, it is known from Dean et al. (1999) that only 3-10 copies of these spike DNA fragments containing the SV40 enhancer are needed to be inserted into a single cell for the risk of insertional mutagenesis to exist,” he said.
This risk posed by millions to billions of small DNA fragments per dose was highlighted last year by cancer genomics scientist Dr Phillip Buckhaults, from the University of South Carolina, who verified McKernan’s DNA contamination findings in his own lab.
In sworn testimony in a South Carolina Senate hearing, Dr Buckhaults explained that by chopping residual DNA fragments into “itty bitty bits“ as part of the filtering process during mod-RNA vaccine production, the vaccine manufacturers “actually increased the hazard of genome modification in the process.”
Insertional mutagenesis (i.e.: insertion of small fragments in the genome) can in turn lead to cancer formation, Dr Speicher explained further via email.
“[That’s] why the p53 gene is so important. The SV40 promoter knocks out p53 and can cause the cell to turn cancerous. We also know from Kevin’s sequencing studies that the whole spike gene can insert into precancerous regions of chromosome 9 and 12,” he said.
DNA contamination in Covid vaccines DOES get into human cells, new evidence shows
Regulators and fact checkers claim that plasmid DNA contamination in the mRNA Covid vaccines can’t change your genomic DNA, but new evidence suggests that it actually can.
The oncogenic risk is highlighted in the U.S. Food and Drug Administration’s (FDA) industry guidance, which states, “There are several potential mechanisms by which residual DNA could be oncogenic, including the integration and expression of encoded oncogenes or insertional mutagenesis following DNA integration.”
Several Moderna patents (here and here) similarly reference the risks of oncogenesis and DNA integration associated with residual DNA.
Dr Speicher’s affidavit references new research conducted by McKernan and molecular biologist Dr Ulrike Kämmerer showing that “integration of the DNA fragments in the Pfizer COVID-19 modRNA vaccine into the human genome is possible.”
“It is important to investigate whether integration can take place in primary cells in the vaccinated population,” he said.
Dr Speicher told me that the next step from here will be to “dive deeper into determining if and where insertional mutagenesis is occurring” in vaccinated people, “including by comparing vaccinated and unvaccinated blood and sperm samples.”
“Sperm will be especially huge because if it is shown that the spike DNA is in in sperm cells and that cell makes an offspring than every cell in the offspring’s body could be a spike factory.”
Cancer genomics scientist Dr Buckhaults has begun his own study to test for genomic integration in mod-RNA vaccines, which he said he hopes will “prove my concerns are unwarranted by accumulating a lot of negative data.”
McKernan intends to formally publish his Pfizer vaccine integration study with Dr Kämmerer and has more experiments in progress to figure out if the integration is occurring in hereditary or non-hereditary chromosomes.
Battle in the court
Dr Speicher’s affidavit will be presented as evidence in the case, Julian Fidge v. Pfizer, Moderna. The plaintiff, Victorian GP and pharmacist Dr Julian Fidge, is seeking an injunction from the Federal Court to stop Pfizer and Moderna from distributing their mod-RNA Covid vaccines.
Dr Fidge alleges that the vaccines contain GMOs, for which Pfizer and Moderna did not obtain the appropriate licence from the Office of the Gene Technology Regulator (OGTR) before distributing the vaccines, a serious criminal offence under the Gene Technology Act (2000).
The OGTR and the TGA deny that the mod-RNA-LNPs and synthetic DNA fragments are GMOs under Australian law, but scientific and legal experts who provided evidence for the lawsuit disagree.
The matter was due to be settled in the Federal Court, but is in a holding pattern while the Federal Court formally investigates a judge who dismissed the case on the matter of standing.
The investigation was initiated in response to a complaint raised by Dr Fidge’s lawyers, which alleges that Justice Helen Rofe concealed her prior professional relationship with one of the defendents, Pfizer, as well as family ties to the biomedical industry, before dismissing the GMO case.
Learn more about the GMO case here.
To support my work, share, subscribe, and/or make a one-off contribution to DDU via my Kofi account. Thanks!
Moderna was contacted for comment on whether this shortcoming has been addressed in its protocol for measuring residual synthetic DNA in its Covid vaccines for submission to regulators, but did not respond before publication deadline.
A draft Clarifax response emailed from Senior HC Biologist/Evaluator Michael Wall to HC Manager of Vaccine Quality Tong Wu states, “Health Canada will continue to work with international regulator partners to achieve harmonisation regarding removal of these sequence elements from the plasmid for future strain changes.”






I think it is safe to assume, from your article, that the TGA will always and forever say "safe and effective". No matter the evidence, no matter the cost.
Great work (again) Rebekah!
So many issues raised with this.
The TGA that discounts this study is the same TGA that bent over backwards to accept Pfizer's heavily doctored initial Covid "vaccine" study despite its gaping flaws.
And considering that the entire concept of mRNA agents encased in lipid nanoparticles with hugely varying quantities of DNA plasmids has been around for 5 minutes, on what grounds does the TGA state they are "safe"?
And on what grounds do they provide a 10ng cut off limit for a "safe" level in a novel treatment?
And where is the curiosity from the medical colleges, AMA, individual doctors, the media, the health departments, CMO's and politicians in at least taking an interest in this?