Behind the scenes: How Freedom of Information works
A case study of the TGA's transparency problem
You’ve got better chances of getting blood from a stone than getting information out of the government agencies if they don’t want to give it to you.
Readers of this Substack know about the difficulties of extracting information from the Therapeutic Goods Administration (TGA) in particular.
From hiding post-vaccine deaths of children, to sending irrelevant information in lieu of the information requested, to publishing documents entirely black with redactions - there are lots of tricks to block the release of information that the government doesn’t want to get out.
In this post, I’ll take you behind the scenes on my most recent request to the TGA under Freedom of Information (FOI) laws so you can see how it’s done.
The request
This FOI request underpinned my latest investigation:
EXCLUSIVE: 35 people died the same day as their Covid shot. Authorities did not investigate.
Australians are routinely assured that deaths arising from Covid vaccination are vanishingly rare, based on the drug safety regulator’s claim that it has identified only 14 deaths linked to vaccination out of more than 70 million doses given. However, new documents released under Freedom of Information (FOI) laws suggest that the public has been misled...
Earlier FOIs by Dr Suzanne Niblett had identified 35 deaths reported to the TGA which occurred on the same day as Covid vaccination - ‘day zero deaths.’
Building on Dr Niblett’s work, on 16 January 2025 I requested:
The latest numbers on day zero deaths (as at 31 December 2024);
Case numbers for all day zero deaths;
a)The Fatal AEFI Assessment Team Meeting Close Out Summary Reports for all day zero deaths (Also known as a ‘causality assessments.’ There should be one for every reported death that has been assessed for causality.). I also requested any other documents pertaining to assessment of causality;
b) Vaccine Safety Investigation Group (VSIG) meeting minutes for the 35 deaths if a VSIG meeting was convened to review the case (A VSIG should be convened in the most serious cases that could change the favourable benefit-risk balance of the vaccine or could threaten public confidence in vaccine safety); and,
The WHO causality assessment classification code assigned by the TGA to each of these deaths.
Start with everything you want, and the agency will usually come back to you to negotiate a narrower scope.
Initial refusal: revision required
Government agencies must acknowledge an FOI request “as soon as practicable” and a decision must be made on the finalised request within 30 days, although the TGA will often ask for extensions at various stages of the process.
On 30 January, the TGA came back saying they would refuse the request unless I could revise it because, “the work involved in processing your request in its current form would substantially and unreasonably divert the resources of the TGA from its other operations due to its voluminous nature.”
This is called a ‘practical refusal’ and is the standard reason given for refusing requests that require multiple documents, especially where the request involves the need for consultation with third parties.
The decision maker advised that around 175 documents had been identified for item #3, and would potentially require consultation with 35 parties.
The decision maker also determined my requests #1, #2 and #4 invalid as “the purpose of the FOI Act is to provide access to documents, not to provide information, undertake bespoke analysis, or answer specific questions.”
This is one of the tricky things about getting information under FOI - you need to figure out which documents contain the information you want, then request those exact documents.
This was actually a problem for multiple people seeking time to death (TTD) information. One colleague was denied his request for TTD information on reported deaths because the TGA said it did not have the “required software” to collate this information from the Database of Adverse Event Notifications (DAEN). This is another type of ‘practical refusal.’
This was tricksy, because the TGA can (and subsequently did) collate this information from its back end system, the Adverse Event Management System (AEMS), which feeds into the DAEN. However, if you don’t word your request in the precisely perfect way, you’ll just get a ‘computer says no’ refusal.
Dr Niblett had to submit multiple separate FOIs to get the information required to finally submit the perfectly worded request to obtain TTD information. You can read about Dr Niblett’s process in her TTD report attached in footnote #3 at the end of this post.
Back to my FOI request. The decision maker did advise that no further ‘day zero’ deaths had been reported since Dr Niblett’s prior FOI request identifying the 35 deaths (as at 5 September 2024). This meant I was able to remove item #1 from my request.
The decision maker refused item #2 because “this information has already been released under FOI-0047,” which is incorrect, as you’ll see in the next section.
For item #4, the decision maker stated,
“I can advise that the vast majority of COVID-19 adverse event reports are included as ‘causality possible’ when they are processed, even when there is very limited information on which to further consider the relationship between the vaccine and adverse event. Reports of death occurring after vaccination have been subjected to the initial causality assessment and have been included in our internal adverse event database as ‘causality possible’ so that they can be included in our safety monitoring activities. As such, even when there is limited information, and even when a coroner or expert panel has concluded it as a death unrelated to COVID-19 vaccination, the report remains included in our safety monitoring data as ‘causality possible.’”
This is obviously very different to the government’s public messaging on vaccine-related deaths, which is that the TGA has only identified 14 causally linked reports, implying that the remainder are just coincidences - a key point made in my subsequent article on the 35 day zero deaths. Sometimes you can glean useful information from the FOI process itself, not just in the documents requested.
The consultation period
Under Australia’s FOI laws you can request consultation for assistance in revising your scope to have the best chances of an FOI request being fulfilled. The consultation period for revision of scope is time-limited to 14 days.
On this occasion, I requested an extension of seven days to the consultation phase because the TGA burned through my consultation time with its slow response times.
During this process I altered my request on some points, and argued others:
I agreed to remove item #1 from my request;
I argued that the decision maker was incorrect about case numbers requested at item #2 having already been released under FOI-0047. No case numbers are provided in FOI 25-0047, and where they are provided in another related FOI (25-0093), they are listed in ascending order and are not linkable in any way to the information provided in FOI 25-0047;
I agreed to reduce the scope of item #3 down to only the single page causality assessment reports of the 35 reported day zero deaths, as well as VSIG meeting minutes and documentation in the cases where VSIG was convened;
I compressed items #2 and #3 together by stipulating that unredacted case numbers would be included on the causality assessment reports; and,
I requested clarification on the TGA’s reluctance to meet my item #4 request: “Could the TGA please clarify - are you saying that no documents exist identifying the WHO coded outcomes for the 35 deaths TTD (time to death) of 0 days?”
By the time I finalised my request, I realised that WHO codes are included on causality assessment reports, so this information request was already covered by item #3. Often, the TGA will send lengthy paras of waffle going around in circles rather than just giving simple clarifications like this.
Further revisions
On 7 February the TGA replied arguing that much of the information I had requested was too sensitive to be released, and may risk re-identification of individuals if triangulated with other publicly available information or media reports.
The TGA also expressed concern over the potential need to consult with bereaved families to obtain permission to release such sensitive information. The TGA argued that “consultation in these circumstances would not only be considered an unreasonable diversion of TGA resources to process, but would also likely cause undue suffering and hardship for those parties consulted.”
The risk of re-identification and the presumed suffering that consultation would cause the bereaved are the TGA’s second and third go-to arguments for denying disclosure of safety surveillance information, after the first line “too voluminous” practical refusal.
On 10 February, I replied, again altering my request on some points, and arguing others:
“I definitely do not accept that families of the deceased don't want to be consulted, as you have suggested. In interviews, families of the deceased generally count the lack of contact from the TGA following their loved ones' deaths as compounding their pain and suffering, and express a desire for better follow up and transparency. This was demonstrated in the case of Natalie Boyce, whose mother called the TGA's lack of communications with her “disgraceful treatment of a grieving mother who could have made a meaningful contribution to their investigations,” (interview with Jab Injuries Australia 13 Feb 2023) and was gratified by the release of FOI 3727, which the TGA initially concealed from the public disclosure log based partly on the claim that "consultation with the families of the deceased was not considered appropriate (Letter to Dr Melissa McCann 24 August 2022).”
“I also do not accept the assertion that it is an unreasonable diversion of time to provide close out reports for deaths occurring on the same day as vaccination. This is a matter of intense public import and interest.”
Some more back and forthing ensued. The TGA persisted in asking me to agree to:
Significantly reduce the number of cases for which you seek documents about (or else risk practical refusal due to volume); and,
The redaction of case numbers and any identifying case details (such as the
reaction terms, age, sex, etc), in order to reduce the chance of identifying individuals.
I emphasised that these requests did not require disclosure of any personal information of TGA staff or meeting attendees.
I allowed that some personal information of day zero death cases could be redacted, but that I expected patient age, gender, case number, and reaction terms/diagnoses to be exempted from redaction as “this information is all currently available on the publicly available DAEN as de-identified case details." The only thing I was seeking was to link this already public information to the day zero death status.
To minimise chances of re-identification, I specifically excluded date of death from the information requested.
Request accepted
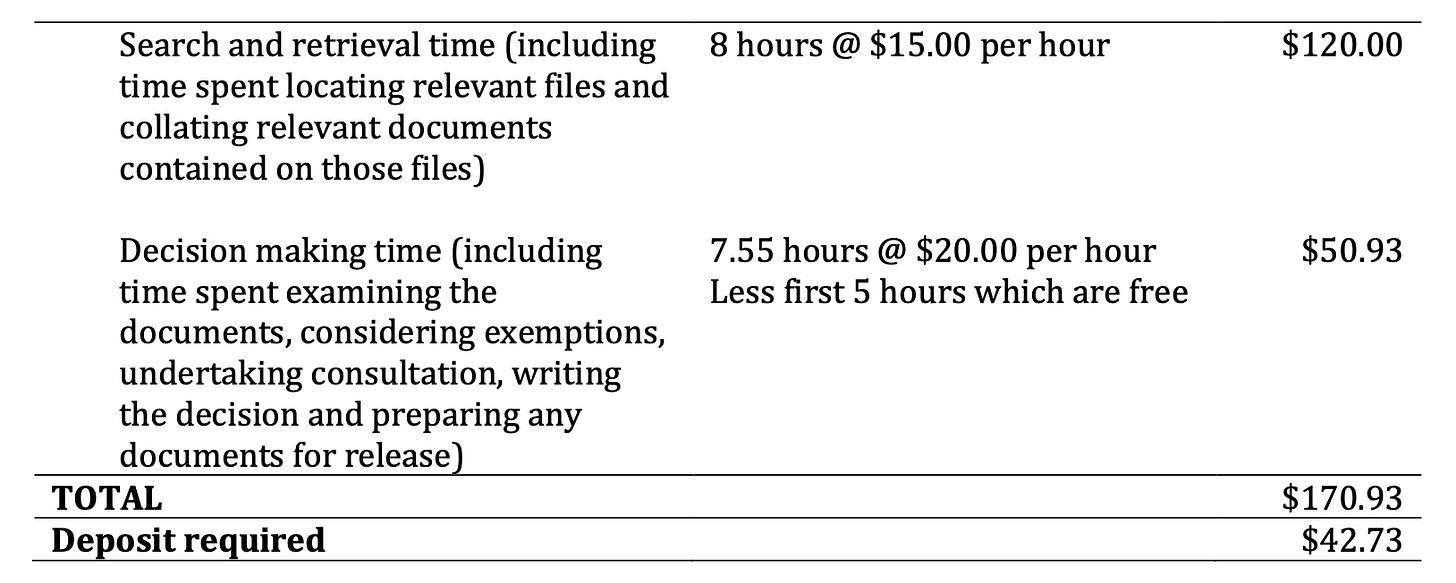
When an FOI request is finally accepted after the consultation phase, the government agency may charge a fee for estimated time spent finding, retrieving, and reviewing the requested documents.
On 14 March I received notice that my request would cost an estimated $170.93 for time spent as detailed in the table below. A deposit is required to start the clock on the 30-day decision timeframe. When the documents are ready for release, the agency reviews the time spent on the request and may adjust the final payment amount accordingly. The remainder must be paid before documents are released.
Notice of decision
On 26 March I received the Notice of Decision on my FOI request, with the instruction to pay the balance of my charges (which had been revised down to $77.27) to secure release of the documents.
In the decision I was informed that I was to be granted “partial” access to the documents, meaning that portions would be redacted, based on Sections 22 and 47F of the Freedom Of Information Act (1982).
Section 22, provides for the redaction of irrelevant information - in this instance, TGA staff names, contact details, and position titles. Fine, I had already stated this information was not required.
Section 47F provides for the redaction of personal information - this was used to justify the redaction of almost every useful detail on causality assessment reports except for the WHO causality classification codes, although you don’t get to see what exactly has been redacted until you pay for and receive the documents.
Information that was redacted with the justification of Section 47F:
The age, gender and state of the deceased;
The brand name of the vaccines;
The medical history of deceased persons;
The cause of death of the deceased; and,
Other personal information about the deceased.
The decision also referred to only 24 documents. There should have been 35 at minimum (the causality assessment reports) plus VSIG minutes.
I had two options:
Don’t pay, don’t receive any documents, and request an internal review of the decision.
Pay, receive the documents, and request an internal review of the decision.
I speculated that the 24 documents would provide some insights nonetheless, so I made my payment. The documents were released to me on 31 March. You can see how heavily redacted they are, here.
As it turned out, these 24 documents did provide some useful information - moreso, the absence of any VSIG minutes and the other 11 causality assessment reports. Read more about that in my article, ‘EXCLUSIVE: 35 people died the same day as their Covid shot. Authorities did not investigate.’
Internal review
After an FOI decision is handed down, you have 30 days to lodge an internal review. Alternatively, you can go straight to the Office of the Australian Information Commissioner (OAIC) to request a review of the decision, but as this office is infamous for its backlog, taking months or even years to process review requests, I decided to go through the internal process with the TGA to start with.
On 7 April, I lodged an internal review request providing the following reasoning:
1. Per item 3 of my communication 21 Feb 2025, my explicitly stated expectation was that case numbers not be redacted from close out forms. However, case numbers have been redacted in the documents provided to me. I request that the case numbers be unredacted.
2. I request that all sections redacted under Section 47F be revised and unredacted please. My reasons for this request are:
a) TGA has previously provided this information unredacted before, so clearly there is an inconsistent standard being applied. See FOI 3727 as example.
b) Based on FOI 3727, my expectation of the extent of any redactions made under S47F was that they would be limited and only strictly necessary. The redactions made to FOI 25-0115 are excessive and unnecessary.
c) As previously stated, I consider the patient age, gender, case number, and reaction terms/diagnoses to fall outside the definition of personal information. Your decision dated 25 March contests that individuals may be identified by members of the public. I wish to know how the TGA arrives at the conclusion. By what means will members of the public make these identifications? A google search yields no media reports of anyone dying on the same day as their vaccination, so triangulation with media reports is not possible in this instance.
d) The TGA appears to apply a double standard on the matter of identification. Media regularly report on adverse events reported to the TGA following use of various medications and supplements in great detail, such as this report in which the victim is not only described, but named.
e) Information that has no relevance to personal privacy has been redacted. For example, vaccine brands. The interpretation of S47F in redacting information of public interest such as the brands of vaccines used in these cases is clearly overbroad.
3. Only 24 Fatal AEFI Assessment close out reports have been provided out of 35 requested. Does this mean that in the remaining 11 cases, no Fatal AEFI Assessment Team meeting was held, and that no corresponding close out summary reports exist? If yes, please confirm. If no, please provide the missing 11 documents.
4. No VSIG meeting minutes have been provided. Does this mean that VSIG was not convened for any of these 35 cases? If yes, please confirm. If no, please provide the missing VSIG minutes.
On 2 May, I received a notice of decision for the internal review.
In short, the decision maker upheld the original decision in full. The main reasons given were that:
"giving access to the information at this time would be contrary to the public interest” (Disclosing the vaccine brands associated with deaths on the same day as vaccination would not be in the public interest? That’s a stretch!);
concerns that I would release the information “to the world at large;”
“information may be subsequently inputted into the Open DAEN website, which would enable any member of the public to practically access and link
this information with other information about these adverse event reports” (ironic, given that Open DAEN was created by citizens to improve access to TGA safety surveillance data from the DAEN, which is so clunky and complex that it is almost unusable even for experienced researchers);
“the disclosure of this information would [not] shed light on the workings of government, nor enhance the accountability or transparency of the obtaining access to reports generated by the TGA;" and,
“there is a lot of information in the media about cases where patients have died following covid-19 vaccination” (despite there being zero reports of patients dying on the same day as their Covid shot, as raised in my review request).
The fact that the decision maker was so concerned at the prospect of me releasing the information “to the world at large,” combined with the inconsistent application of the S47F exemption (the same unredacted information I’m requesting has been released for other vaccine reports, and for Covid vaccine death reports under FOI 3727) is suggestive that there was some political motivation to blocking disclosure of this information.
FOI 3727 resulted in a fair bit of negative publicity for the TGA, as it exposed the fact that the regulator had hidden reports of children’s post-vaccine deaths from the public, justifying this opaque conduct with the insipid excuse that “disclosure of the documents could undermine public confidence.”
Under Section 11B of the FOI Act, it is expressly stated that government agencies must not block disclosure of information for fear of embarrassment to the government, anticipated misinterpretations, or the prospect of public confusion and unnecessary debate. However, as the decision maker did not outright admit to any political motivation, it is hard to hold the TGA to account on this.

Regardless, the decision did provide some more useful information:
Confirmation that only 24 out of 35 day zero deaths have a corresponding causality assessment close out summary report; and,
Confirmation that none of the day zero death cases were referred to VSIG.
Amusingly, the decision maker spent one and a half pages deliberating whether to exclude THEIR OWN NAME from the decision notice. This person decided to remain anonymous because the disclosure of his or her name “would have a substantial adverse effect on the management of personnel by the Commonwealth" including being "subjected to harassment, intimidation and threats by members of the public."
The “Authorised decision maker” formed this view “because I am aware of previous instances of harassment that FOI decision makers and TGA staff have received in relation to matters relating to the Covid-19 vaccines, including in relation to matters that are similar to your FOI request.”
I wonder if this is a reference to published articles naming and rebutting decision makers’ reasoning, which is not harassment? Or, have TGA staff experienced real actual harassment, like stalking, doxxing, or barrages of abusive messages? It should go without saying, but dear readers, please don’t harass bureaucrats.
The internal review is the end of the line for dealing with the TGA on an FOI request. Your next option is to lodge a case with the OAIC, which is the national regulator for privacy and freedom of information.
Information Commissioner review
Unfortunately, the OAIC is notoriously backlogged.
I’ve had an Information Commissioner (IC) review request sitting in the OAIC queue since December last year. They’re so overwhelmed that they don’t even respond to progress inquiries. To the best of my knowledge, six months after my review request was lodged, the OAIC hasn’t even begun the process of reviewing it.
This is unsurprising. The report from a 2023 Senate inquiry into Australia’s FOI system noted that, “during proceedings, the OAIC revealed that around 80 per cent of the 325 IC reviews lodged with the OAIC in 2020 had not been allocated to a reviewer.”
This report, and most media commentary, agree that the OAIC is chronically underfunded and understaffed.
The former FOI Commissioner, Leo Hardiman, resigned from his post (which operates under the OAIC umbrella) in March 2023, stating that his position was untenable because changes that would need to improve timeliness of IC reviews were “not within the powers conferred on me as FOI Commissioner.”
That leaves Australia with a hamstrung information regulator. Government agencies are therefore fairly free to push the boundaries of FOI refusals and redactions - and they do.
I tend not to lodge IC review requests unless I genuinely think I have a chance of getting a decision overturned. It’s rather time consuming to lodge the review request, and I don’t wish to add to the backlog with reviews that are unlikely to go anywhere. I’m still deciding if I’ll take the matter further on this particular FOI request.
Broken FOI system
The purpose of the FOI Act is to ensure that Australians have the right to access information held by the federal government, including ministers and most agencies (state and territory governments have their own similar frameworks).
It is supposed to promote transparency and accountability, and, in Hardiman’s words, to provide “one check on the integrity and apolitical nature of the Australian Public Service.”
However, the system has many shortcomings. Some of the problems raised in submissions to the 2023 Senate inquiry included:
Perverse outcomes that encourage government agencies to ignore processing times and have the effect of nurturing secrecy;
The ability of the government to hide failures, mistakes, and corruption;
Substantial delays in processing FOI requests, which can impact the news value of media stories, and the ability of academics to access information for research;
Inconsistencies in exemptions and unwarranted redactions of key information;
‘Obstructionist tactics’ and ‘administrative torture’ faced by journalists pursuing FOI requests;
The higher the level of political importance of a document that is subjected to an FOI request, the harder it is to get a response from the decision-making agency; and,
A cultural problem whereby agency bureaucrats tend to use FOI provisions to block release of information (often excessively or inappropriately) rather than taking a pro-disclosure approach.
One of the committee’s recommendations, a strategic assessment of the OAIC, has since been completed, though the resulting corporate plan has yet to be fully implemented.
A more muscular regulator and a culture change would be welcome, but neither are likely to occur in the near future - there is not the funding or the political will.
Nevertheless, as you can see from this tour behind the scenes, there is still value to be mined from the FOI process, broken as it is.
You just need persistence, the patience of a saint, laser precision with articulating your request, and very good record keeping skills.
To support my work, share, subscribe, and/or make a one-off contribution to my Kofi account. Thanks!






Transparency and accountability in government? Free country? Democracy?
You're a champion for negotiating their muddy obstacle course. Apparently they don't like champions. But history does.
I had one encounter with NSW's GIPA in 2021 when I wanted justification for being unable to resume bus driving duties due to a mask exemption policy by NSW Transport (fortunately still employed at the time and confined to 'light duties', which was mostly bus refuelling and parking in the depot).
I had realised by then the legalese required to extract blood from the stone that is Government. My GIPA was as follows (22T-0598 in the disclosure log)
"Between and/or about the 16th through 23rd of August, 2021, Transport for NSW has requested that bus drivers possessing valid medical exemptions to mandatory face coverings (masks) cannot drive in public-facing roles. Please see attached email from my former Depot Supervisor for evidence that this policy is currently in place at NSW public service bus depots.
I am hereby requesting the following information from TfNSW concerning the policy decision ("decision") enforced at that time that public bus drivers with valid medical exemptions to mandatory face coverings are prohibited from driving in-service buses:
1. The reasons, grounds and/or rationale supporting the decision;
2. The data supporting the decision, including, but by no means limited to:
a) Reports and/or statistics of COVID-19 acquisition/transmission on NSW public bus services, especially amongst unmasked drivers with medical exemptions;
b) medical and/or scientific studies demonstrating that face coverings are an effective control for the prevention of respiratory illness, especially in, but not necessarily limited to, the enclosed and air-conditioned setting of a bus;
c) if available, a policy-grade report and/or data from an expert (physicist) in fluid dynamics demonstrating the risk of viral nanoparticles suspended in oral or nasal fluid traversing fluid air in an air-conditioned bus environment and the effectiveness of face coverings in mitigating that risk;
3. The risk assessment report supporting the decision;
4. The data analysis report which informed the construction of the risk assessment.
5. Emails and/or communications from and/or between TfNSW staff involved in the consulting, making and implementing of the decision to prohibit bus drivers with valid mask exemptions from operating in-service.
Any documents (including emails and/or communications) which answer the above requests can be supplied in PDF form to the email address provided in this request.”
I eventually got a full release without cost (as the assessing officer waived the costs due to my personal interest in the industry)... mostly consisting of emails surrounding the drafts of the risk assessment and eventual policy statement. It was the most underwhelming example of policy by authoritarianism.
ZERO EVIDENCE/DOCUMENTS PROVIDED FOR Q.2.
Strangely, TfNSW quietly dumped the policy and we returned to driving duties in September 2021.